No notifications yet
Vaxxas Expands Global Intellectual Property Portfolio for Exclusive Manufacture and Sale of Needle-Free Vaccination Technology
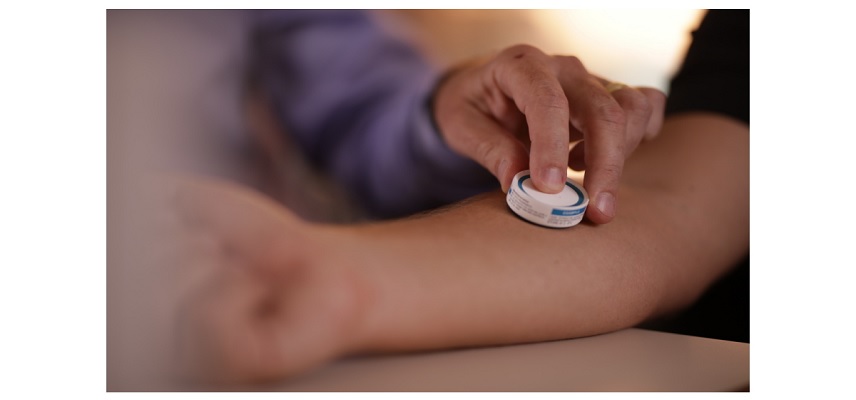
Vaxxas, a clinical-stage biotechnology company, has expanded its global intellectual property portfolio to 42 patents, supporting its exclusive claim to manufacture and sell its proprietary vaccination technology, the high-density microarray patch (HD-MAP), in the United States, Europe, Asia and Australia.
Vaxxas is scaling up to manufacture and distribute the world’s first commercially available vaccine patches from its global headquarters and state of the art biomedical facility in Brisbane.
Vaxxas’ HD-MAP technology has completed five successful Phase I clinical trials involving over 500 participants with vaccines that address some of the world’s biggest health challenges including COVID-19, flu, and measles and rubella.
Vaxxas is currently conducting its first US IND-enabled Phase I clinical study for a pre-pandemic influenza vaccine involving 258 participants, with funding from the United States Biomedical Advanced Research and Development Authority (BARDA).
OneVentures Innovation Fund I and Brandon BioCatalyst are supported by the Australian Government’s Innovation Investment Fund (IIF) program. The IIF is an Australian Government venture capital initiative that provides investment capital and managerial expertise through licensed venture capital fund managers to investee companies. Learn more at OneVentures and Brandon Capital.
- Previous studies have also shown the safety and tolerability of Vaxxas’ HD-MAP for use in vaccine delivery and inducing equal or greater immune responses to injected vaccines at lower doses.
- Compared with needle and syringe systems, HD-MAP vaccines are designed to be easier to administer and have potential use in future pandemic responses.

Sick and tired of always wondering if you are being asked to pay the right price for your APIs? This empowers you with the answers you need to make the right decisions in the Global API market.
Chemxpert Database is one of the biggest and most comprehensive directories of pharma and chemicals, manufacturers, suppliers and information. Provided with current information on prices, demand and transactions, it gives you instant feedback on whether you are buying what is right and at the right time.
Start using market intelligence today and allow yourself to be in control in the API market.
Check it out today and make more informed sourcing decisions! Learn More!
